AN ALLOA mum is urging the Scottish Medicines Consortium (SMC) to approve a drug which has given her son "the chance at life" for use in the NHS in Scotland.
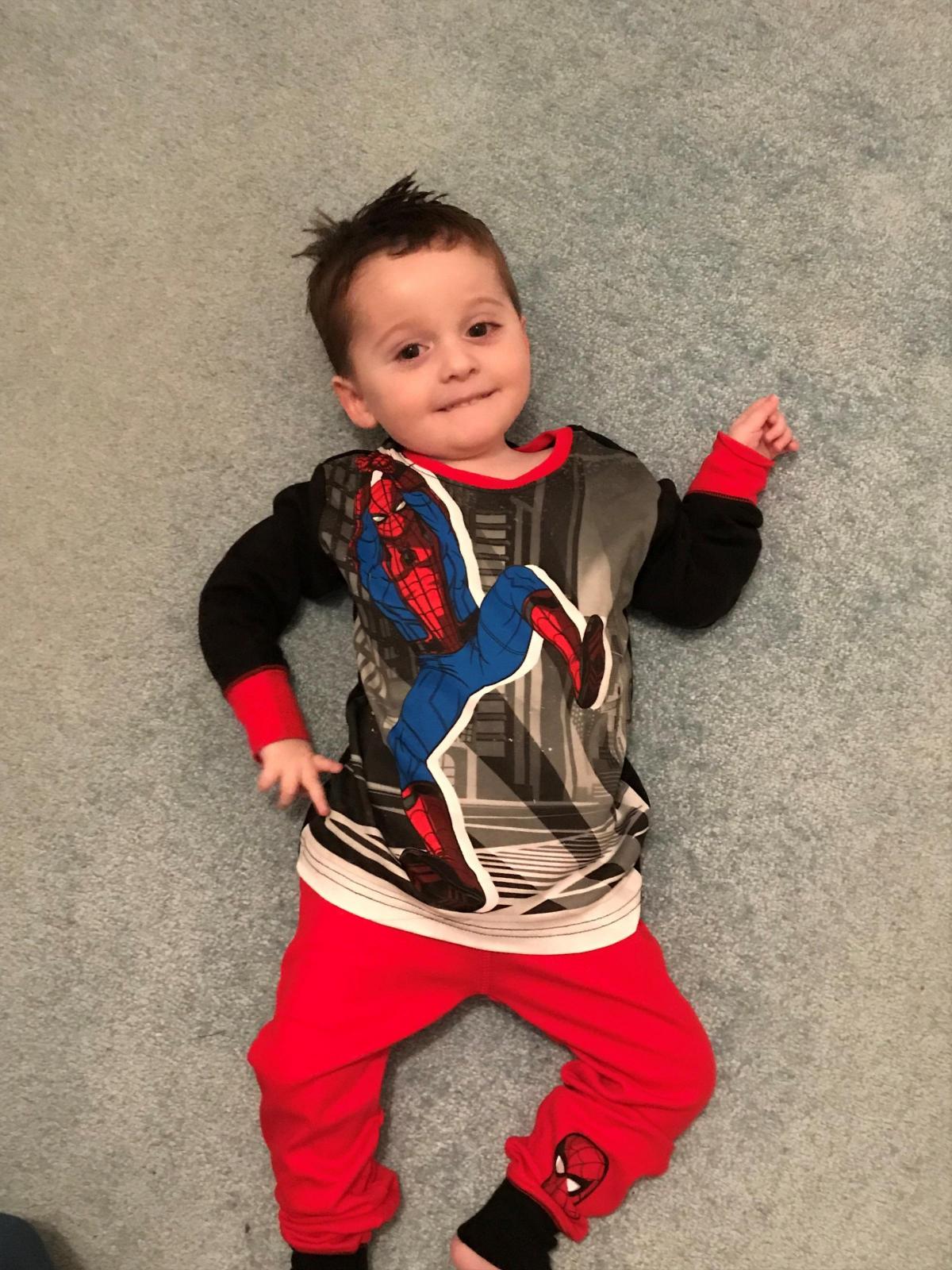
Mum Amy Cameron said when Zac, now two, was diagnosed with the rare muscle-wasting condition spinal muscular atrophy (SMA) Type 1 her world began to fall apart.
A "cruel condition", the future for the family looked increasingly bleak, but all this changed when they heard about Spinraza and their spirits were instantly lifted.
The first and only treatment for patients with the condition, the SMC will make its decision on the approval of the medication on May 7.
Ahead of this, the local family is encouraging the body to "do the right thing" to help those in need of a lifeline.
Amy, 30, said: “While it’s not a cure, it is buying us valuable time with Zac and enabling him to reach milestones we never thought would be possible, such as picking up his cup for a drink and moving his legs.
“Zac has so much to offer the world and this is the only treatment that is giving him the chance at life.
“As parents, my husband and I are determined to secure the happiest life possible for Zac and his brother.
“Life is for living and making memories and we will face whatever challenges lie ahead together as a family.
“The SMC must do the right thing and approve Spinraza. It has given our family renewed hope for a brighter future for Zac and there are many other families out there who desperately need this lifeline.
“We hope all children with SMA will get access to the drug.”
The decision to be made in May follows a special meeting on April 3, where families affected by the condition made a final plea to the SMC to approve Spinraza for use on the NHS in Scotland.
Muscular Dystrophy UK is warning that unless it goes ahead, those who could benefit from a proven treatment will be made to suffer needlessly.
Robert Meadowcroft, chief executive, said: “SMA can be devastating, and Spinraza offers families a rare glimmer of hope.
“Its most severe form means parents have to see their child gradually lose their ability to crawl, move, breathe and swallow while there is a treatment out there which could help.
“Nothing can prepare you for the emotional turmoil this causes.”
The charity said Spinraza proved so effective in a clinical trial for children with SMA Type 1 that it was stopped early so that all youngsters affected could access the treatment.
This is now being delivered to children in Scotland via a special compassionate access scheme, with the drug being provided free by pharmaceutical manufacturer Biogen and the NHS in Scotland funding the costs of administering the treatment.
However, it is a temporary initiative, and only supports a small number of those with SMA who could benefit.
Mr Meadowcroft said: “Spinraza is not a cure, but it can buy families time to spend with their loved ones.
“Some children who have received Spinraza have seen their muscle strength improve and have already lived long enough to crawl, and even walk. For parents, this is priceless.
“The SMC has a chance to hand patients the lifeline they so desperately need.
“If they squander this opportunity to bring hope into the lives of people affected by muscle-wasting conditions, families will be made to pay a very heavy price.”
Find out more on Muscular Dystrophy UK's Fast Track campaign to speed up access to treatment at musculardystrophyuk.org/fast-track




Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules here